TECHNOLOGY
MemoryStim Technology
MemoryStim is preparing to launch a new head and body wearable which will be the first body > brain-stimulator designed to activate specific memory-enhancing protein expressions on-demand via precise patented and patent-pending bioelectric signaling sequences. The bioelectric signaling sequences in pulses are delivered non-invasively to the parts of the brain responsible for learning and memory. The wearable device under development is intended to improve cognitive performance and memory.
Some of the patented and patent-pending intended target memory-enhancing protein expressions of the MemoryStim device include:
- IGF1 and IGF2 – The IGFs cross the blood-brain barriers2 and have endocrine roles in brain.
- SDF and PDGF – stem cell homing factors
- GDF10 and GDF11 – brain regeneration promoting proteins
- VEGF, SDF1, PDGF, EGF, HIFa, eNOS – angiogenesis (new blood vessel formation) promoting proteins via a tightly regulated process that involves the proliferation, migration, differentiation, and organization of endothelial cells into new functional microvessels
- Sonic Hedgehog – results suggest that fear learning induces Shh signaling activation in the amygdala, which promotes neurogenesis and fear memory formation
- Klotho – a longevity factor that enhances brain cognition
- Neurogranin Ngrn and FMRP – memory formation is associated with a singular increase in mRNA levels of the gene Ngrn, which codes for a protein called neurogranin further investigation revealed that another protein, called FMRP, is also critical

The researchers discovered that memory formation is associated with a singular increase in mRNA levels of the gene Ngrn, which codes for a protein called neurogranin, which was first linked to memory formation in 2017.
When they blocked production of neurogranin in mice, the rodent struggled to form new memories when encountering a new location. If neurogranin was added back in, this effect was reversed.
Thus, formation of experience-related memories appears to rely on rapid production of high levels of neurogranin, but further investigation revealed that another protein, called FMRP, is also critical.
References
IGFs
Insulin-like Growth Factor-1 (IGF-1) Improves both Neurological Motor and Cognitive Outcome Following Experimental Brain Injury
https://www.sciencedirect.com/science/article/abs/pii/S0014488697966292
Abstract
We evaluated the efficacy of insulin-like growth factor-1 (IGF-1) in attenuating neurobehavioral deficits following lateral fluid percussion (FP) brain injury. Male Sprague–Dawley rats (345–425 g,n = 88) were anesthetized and subjected to FP brain injury of moderate severity (2.4–2.9 atm). In Study 1, IGF-1 (1.0 mg/kg,n = 9) or vehicle (n = 14) was administered by subcutaneous injection at 15 min postinjury and similarly at 12-h intervals for 14 days. In animals evaluated daily for 14 days, IGF-1 treatment attenuated motor dysfunction over the 2-week period (P < 0.02). In Study 2, IGF-1 (4 mg/kg/day,n = 8 uninjured,n = 13 injured) or vehicle (n = 8 uninjured,n = 13 injured) was administered for 2 weeks via a subcutaneous pump implanted 15 min postinjury. IGF-1 administration was associated with increased body weight and mild, transient hypoglycemia which was more pronounced in brain-injured animals. At 2 weeks postinjury (P < 0.05), but not at 48 h or 1 week, brain-injured animals receiving IGF-1 showed improved neuromotor function compared with those receiving vehicle. IGF-1 administration also enhanced learning ability (P < 0.03) and memory retention (P < 0.01) in brain-injured animals at 2 weeks postinjury. Taken together, these data suggest that chronic, posttraumatic administration of the trophic factor IGF-1 may be efficacious in ameliorating neurobehavioral dysfunction associated with traumatic brain injury.
Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice.
https://europepmc.org/article/PMC/4287930
a
Abstract
Insulin-like growth factor 2 (IGF2) was recently found to play a critical role in memory consolidation in rats and mice, and hippocampal or systemic administration of recombinant IGF2 enhances memory. Here, using a gene therapy-based approach with adeno-associated virus (AAV), we show that IGF2 overexpression in the hippocampus of aged wild-type mice enhances memory and promotes dendritic spine formation. Furthermore, we report that IGF2 expression decreases in the hippocampus of patients with Alzheimer’s disease, and this leads us to hypothesize that increased IGF2 levels may be beneficial for treating the disease. Thus, we used the AAV system to deliver IGF2 or IGF1 into the hippocampus of the APP mouse model Tg2576 and demonstrate that IGF2 and insulin-like growth factor 1 (IGF1) rescue behavioural deficits, promote dendritic spine formation and restore normal hippocampal excitatory synaptic transmission. The brains of Tg2576 mice that overexpress IGF2 but not IGF1 also show a significant reduction in amyloid levels. This reduction probably occurs through an interaction with the IGF2 receptor (IGF2R). Hence, IGF2 and, to a lesser extent, IGF1 may be effective treatments for Alzheimer’s disease.
PDGF
PDGF-stimulated cell proliferation and migration of human arterial smooth muscle cells: Colocalization of PDGF isoforms with glycosaminoglycans
https://www.sciencedirect.com/science/article/abs/pii/S1357272507001677
Abstract
Platelet-derived growth factor (PDGF) has been implicated in vascular smooth muscle cell proliferation and migration, a key process in vascular disease. PDGF is a family of dimeric isoforms of structurally related A-, B-, C- and D-chains that bind to PDGF receptors. PDGF A- and B-chains occur with and without basic C-terminal amino acid extensions as long (AL and BL) and short (AS and BS) isoforms. This basic sequence has been implicated as a cell retention signal through binding to glycosaminoglycans, especially to heparan sulfate. The aim of this study was to evaluate the biological relevance of PDGF interaction with glycosaminoglycans on the PDGF function in human arterial smooth muscle cells (hASMC). Here, we show that long PDGF isoforms showed greater affinity for hASMC cell surface and that they also presented more colocalization with heparan and chondroitin sulfates present on hASMC cell membrane than did short isoforms. Furthermore, all PDGF isoforms colocalized more with heparan sulfate than with chondroitin sulfate and there was little colocalization between heparan and chondroitin sulfate. PDGF-stimulated hASMC activation of DNA synthesis and directed migration (chemotaxis) was also examined. The isoform PDGF-BBS induced maximal proliferation and migration of hASMC. Collagen-I coating significantly increased hASMC motility towards PDGF isoforms, and particularly toward PDGF-BBS. These results strongly support the notion that cell surface glycosaminoglycans are not essential for receptor-mediated activity of PDGF and may contribute basically to the retention and accumulation of long PDGF isoforms.
SONIC HEDGEHOG
Learning induces sonic hedgehog signaling in the amygdala which promotes neurogenesis and long-term memory formation.
https://www.ncbi.nlm.nih.gov/pubmed/25522410
Hung HC1, Hsiao YH1, Gean PW2.
Author information
Abstract
BACKGROUND:
It is known that neurogenesis occurs throughout the life mostly in the subgranular zone of the hippocampus and the subventricular zone of the lateral ventricle. We investigated whether neurogenesis occurred in the amygdala and its function in fear memory formation.
METHODS:
For detection of newborn neurons, mice were injected intraperitoneally with 5-bromo-2′-deoxyuridine (BrdU) 2h before receiving 15 tone-footshock pairings, and newborn neurons were analyzed 14 and 42 days after training. To determine the relationship between neurogenesis and memory formation, mice were given a proliferation inhibitor methylazoxymethanol (MAM) or a DNA synthesis inhibitor cytosine arabinoside (Ara-C). To test whether sonic hedgehog (Shh) signaling was required for neurogenesis, Shh-small hairpin-interfering RNA (shRNA) was inserted into a retroviral vector (Retro-Shh-shRNA).
RESULTS:
The number of BrdU(+)/Neuronal nuclei (NeuN)(+) cells was significantly higher in the conditioned mice, suggesting that association of tone with footshock induced neurogenesis. MAM and Ara-C markedly reduced neurogenesis and impaired fear memory formation. Shh, its receptor patched 1 (Ptc1), and transcription factor Gli1 protein levels increased at 1 day and returned to baseline at 7 days after fear conditioning. Retro-Shh-shRNA, which knocked down Shh specifically in the mitotic neurons, reduced the number of BrdU(+)/NeuN(+) cells and decreased freezing responses.
CONCLUSIONS:
These results suggest that fear learning induces Shh signaling activation in the amygdala, which promotes neurogenesis and fear memory formation.
Neurogranin and FMRP
Memories are made of this: two proteins
https://cosmosmagazine.com/biology/memories-are-made-of-this-two-proteins
Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers.
https://www.ncbi.nlm.nih.gov/pubmed/28939668
Abstract
OBJECTIVE:
To determine the association between synaptic functioning as measured via neurogranin in CSF and cognition relative to established Alzheimer disease (AD) biomarkers in neurologically healthy older adults.
METHODS:
We analyzed CSF concentrations of neurogranin, β-amyloid (Aβ42), phosphorylated tau (p-tau), and total tau (t-tau) among 132 neurologically normal older adults (mean 64.5, range 55-85), along with bilateral hippocampal volumes and a measure of episodic memory (Auditory Verbal Learning Test, delayed recall). Univariable analyses examined the relationship between neurogranin and the other AD-related biomarkers. Multivariable regression models examined the relationship between neurogranin and delayed recall, adjusting for age and sex, and interaction terms (neurogranin × AD biomarkers).
RESULTS:
Higher neurogranin concentrations were associated with older age (ρ = 0.20, p = 0.02), lower levels of p-tau and t-tau, and smaller hippocampal volumes (p < 0.03), but not with CSF Aβ42 (p = 0.18). In addition, CSF neurogranin demonstrated a significant relationship with memory performance independent of the AD-related biomarkers; individuals with the lowest CSF neurogranin concentrations performed better on delayed recall than those with medium or high CSF neurogranin concentrations (p < 0.01). Notably, CSF p-tau, t-tau, and Aβ42 and hippocampal volumes were not significantly associated with delayed recall scores (p > 0.40), and did not interact with neurogranin to predict memory (p > 0.10).
CONCLUSIONS:
Synaptic dysfunction (assessed via neurogranin) may be an early pathologic process in age-related neurodegeneration, and a sensitive marker of age-related cognitive abilities, potentially preceding or even acting independently from AD pathogenesis. Synaptic functioning may be a useful early marker of cognitive aging and possibly a target for future brain aging interventions.
SDF1
The Role of SDF-1/CXCR4/CXCR7 in Neuronal Regeneration after Cerebral Ischemia
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5662889/
Abstract
Stromal cell-derived factor-1 is a chemoattractant produced by bone marrow stromal cell lines. It is recognized as a critical factor in the immune and central nervous systems (CNSs) as well as exerting a role in cancer. SDF-1 activates two G protein-coupled receptors, CXCR4 and CXCR7; these are expressed in both developing and mature CNSs and participate in multiple physiological and pathological events, e.g., inflammatory response, neurogenesis, angiogenesis, hematopoiesis, cancer metastasis, and HIV infection. After an ischemic stroke, SDF-1 levels robustly increase in the penumbra regions and participate in adult neural functional repair. Here we will review recent findings about SDF-1 and its receptor, analyse their functions in neurogeneration after brain ischemic injury: i.e., how the system promotes the proliferation, differentiation and migration of neural precursor cells and mediates axonal elongation and branching.
Stromal Cell-Derived Factor 1 Protects Brain Vascular Endothelial Cells from Radiation-Induced Brain Damage
Abstract
Stromal cell-derived factor 1 (SDF-1) and its main receptor, CXC chemokine receptor 4 (CXCR4), play a critical role in endothelial cell function regulation during cardiogenesis, angiogenesis, and reendothelialization after injury. The expression of CXCR4 and SDF-1 in brain endothelial cells decreases due to ionizing radiation treatment and aging. SDF-1 protein treatment in the senescent and radiation-damaged cells reduced several senescence phenotypes, such as decreased cell proliferation, upregulated p53 and p21 expression, and increased senescence-associated beta-galactosidase (SA-β-gal) activity, through CXCR4-dependent signaling. By inhibiting extracellular signal-regulated kinase (ERK) and signal transducer and activator of transcription protein 3 (STAT3), we confirmed that activation of both is important in recovery by SDF-1-related mechanisms. A CXCR4 agonist, ATI2341, protected brain endothelial cells from radiation-induced damage. In irradiation-damaged tissue, ATI2341 treatment inhibited cell death in the villi of the small intestine and decreased SA-β-gal activity in arterial tissue. An ischemic injury experiment revealed no decrease in blood flow by irradiation in ATI2341-administrated mice. ATI2341 treatment specifically affected CXCR4 action in mouse brain vessels and partially restored normal cognitive ability in irradiated mice. These results demonstrate that SDF-1 and ATI2341 may offer potential therapeutic approaches to recover tissues damaged during chemotherapy or radiotherapy, particularly by protecting vascular endothelial cells.
GDF10 and GDF11
Human rGDF-11 counteracts age-related short-term memory impairments in middle-aged mice
https://curve.carleton.ca/system/files/etd/6d272324-f550-4e4a-9503-47dc43d21256/etd_pdf/7d9569556ad91c020725899a6a8845e6/zhang-humanrgdf11counteractsagerelatedshortterm.pdf
Abstract
In humans, visuospatial memory begins to decline as early as the mid-30s, yet the mechanisms involved in this phenomenon are poorly understood. Recent research suggests that growth differentiation factor-11 (GDF-11) can have a beneficial impact on cognitive ability in old age. The mechanisms mediating this effect are unclear and there is currently no information regarding potential impact of GDF-11 on cognitive ability in the middle age years. The goal of this thesis was to explore the effects of GDF-11 treatment on the cognitive ability in middle-aged mice. Young mice and middle aged mice were treated with GDF-11 and the impact on short term memory was evaluated. The data showed significant improvement in the performance of visual memory tasks and increased neurogenesis in middle-aged mice. Taken together, these results suggest that GDF-11 is a promising candidate for combating the age-related cognitive decline associated with middle age.
Growth Differentiation Factor 11 treatment leads to neuronal and vascular improvements in the hippocampus of aged mice
https://www.nature.com/articles/s41598-018-35716-6
Abstract
Aging is the biggest risk factor for several neurodegenerative diseases. Parabiosis experiments have established that old mouse brains are improved by exposure to young mouse blood. Previously, our lab showed that delivery of Growth Differentiation Factor 11 (GDF11) to the bloodstream increases the number of neural stem cells and positively affects vasculature in the subventricular zone of old mice. Our new study demonstrates that GDF11 enhances hippocampal neurogenesis, improves vasculature and increases markers of neuronal activity and plasticity in the hippocampus and cortex of old mice. Our experiments also demonstrate that systemically delivered GDF11, rather than crossing the blood brain barrier, exerts at least some of its effects by acting on brain endothelial cells. Thus, by targeting the cerebral vasculature, GDF11 has a very different mechanism from that of previously studied circulating factors acting to improve central nervous system (CNS) function without entering the CNS.
Biologists have found a gene that controls brain repair – GDF10
https://www.extremetech.com/extreme/217094-biologists-have-found-a-gene-that-controls-brain-repair
KLOTHO
Peripheral Elevation of a Klotho Fragment Enhances Brain Function and Resilience in Young, Aging, and α-Synuclein Transgenic Mice
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5816951/
Klotho is a longevity factor associated with cognitive enhancement when genetically and widely overexpressed over the lifetime of mice. Leon et al. show that peripheral delivery of a klotho fragment, αKL-F, acutely enhances cognition and neural resilience in young, aging, and disease model mice, establishing its therapeutic relevance and dissecting its underlying mechanisms.
Life Extension Factor Klotho Enhances Cognition
Highlights
•
KLOTHO variant elevates klotho levels and is associated with enhanced human cognition
•
Elevation of klotho in mice enhances normal cognition, independent of age
•
Klotho elevation leads to greater synaptic GluN2B (NMDAR subunit) levels and plasticity
•
GluN2B blockade abolishes klotho-mediated effects on NMDAR functions and cognition
Summary
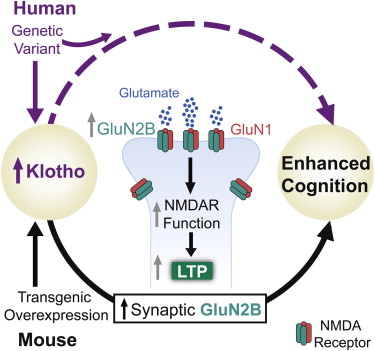
Aging is the primary risk factor for cognitive decline, an emerging health threat to aging societies worldwide. Whether anti-aging factors such as klotho can counteract cognitive decline is unknown. We show that a lifespan-extending variant of the human KLOTHO gene, KL-VS, is associated with enhanced cognition in heterozygous carriers. Because this allele increased klotho levels in serum, we analyzed transgenic mice with systemic overexpression of klotho. They performed better than controls in multiple tests of learning and memory. Elevating klotho in mice also enhanced long-term potentiation, a form of synaptic plasticity, and enriched synaptic GluN2B, an N-methyl-D-aspartate receptor (NMDAR) subunit with key functions in learning and memory. Blockade of GluN2B abolished klotho-mediated effects. Surprisingly, klotho effects were evident also in young mice and did not correlate with age in humans, suggesting independence from the aging process. Augmenting klotho or its effects may enhance cognition and counteract cognitive deficits at different life stages.
Graphical Abstract
Longevity hormone boosts memory and protects against brain aging in mice
Mice treated with klotho do better on tests of memory and motor skills
https://www.sciencedaily.com/releases/2017/08/170808150006.htm
Klotho controls the brain–immune system interface in the choroid plexus
https://www.pnas.org/content/115/48/E11388
Significance
Global depletion of klotho accelerates aging, whereas klotho overexpression counteracts aging-related impairments. Why klotho is expressed at much higher levels in the choroid plexus than in other brain regions is unknown. We demonstrate in mice that aging is associated with klotho depletion in the choroid plexus. Reducing klotho selectively within the choroid plexus triggered inflammation within this structure and enhanced activation of innate immune cells within an adjacent brain region following a peripheral immune challenge. In cell culture, we identified a signaling pathway by which klotho suppresses activation of macrophages. Our findings shed light on klotho functions in the choroid plexus and provide a plausible mechanism by which klotho depletion from this structure promotes brain inflammation during the aging process.
Abstract
Located within the brain’s ventricles, the choroid plexus produces cerebrospinal fluid and forms an important barrier between the central nervous system and the blood. For unknown reasons, the choroid plexus produces high levels of the protein klotho. Here, we show that these levels naturally decline with aging. Depleting klotho selectively from the choroid plexus via targeted viral vector-induced knockout in Klothoflox/flox mice increased the expression of multiple proinflammatory factors and triggered macrophage infiltration of this structure in young mice, simulating changes in unmanipulated old mice. Wild-type mice infected with the same Cre recombinase-expressing virus did not show such alterations. Experimental depletion of klotho from the choroid plexus enhanced microglial activation in the hippocampus after peripheral injection of mice with lipopolysaccharide. In primary cultures, klotho suppressed thioredoxin-interacting protein-dependent activation of the NLRP3 inflammasome in macrophages by enhancing fibroblast growth factor 23 signaling. We conclude that klotho functions as a gatekeeper at the interface between the brain and immune system in the choroid plexus. Klotho depletion in aging or disease may weaken this barrier and promote immune-mediated neuropathogenesis.